脑未来科普 |让下丘脑告诉你吃还是不吃
|
|
常言道,过犹不及,美食亦是如此。近几十年来,随着全球饮食结构的改变,高脂、高糖等食物的消耗不断增加(图1),超重、肥胖已发展成当今社会流行病。2017年《全球疾病负担》(global burden of disease)数据显示,全球有超过400万人因超重或肥胖而死亡。从1975年至2016年,全球5-19岁超重或肥胖儿童和青少年的患病率从4%上升到18%,增长超4倍。超重、肥胖带来的不仅是审美问题,同时也是引发重症疾病的风险因素,如II型糖尿病、心脏病、中风和某些类型的癌症如乳腺癌和结肠癌等。 那么人体是通过何种方式来调节摄食进而影响体重的呢?
|

图1. 高能量食物(图片来自必应)
饮食的激素调节机制
我们的身体对体重的调控包括短期调节和长期调节。例如,由消化道产生的肽激素,即胃饥饿素(ghrelin)、酪酪肽(peptide tyrosine tyrosine, PYY)和胆囊收缩素(cholecystokinin, CCK),与体重的短期调节有关。而瘦素(leptin)及胰岛素(insulin),被认为是体重长期维持的关键激素(图2)。在中枢神经系统中,下丘脑在摄食调节和能量稳态中发挥至关重要的作用,瘦素是下丘脑感知营养状态进而调节食物摄入和能量平衡的主要信号。下丘脑的亚核团弓状核(arcuate nucleus, ARC)表达两类活性相反的神经元,分别是前阿黑皮素原(pro-opiomelanocortin,POMC)神经元(可产生α促黑激素)以及agouti相关肽(agouti-related peptide, AgRP)神经元(可产生神经肽Y),感知脑内血液循环中胰岛素、瘦素等激素的变化进而被兴奋或者抑制,两类神经元整合信号后最终将信息发送到“饱食中枢”—脑干的孤束核(nucleus tratus solitarius, NTS),改变身体的饱腹状态,并在整体水平上调节食物摄入和能量消耗(图3)。例如,当脂肪和瘦素水平下降时,AgRP神经元被激活,POMC神经元被抑制,饥饿感增加,其最终效应是促进摄食增加进而导致体重增加;与之相反,脂肪和瘦素水平的上升则会导致AgRP神经元的抑制以及POMC神经元的激活,饱腹感增加,从而导致摄食减少进而体重减轻。
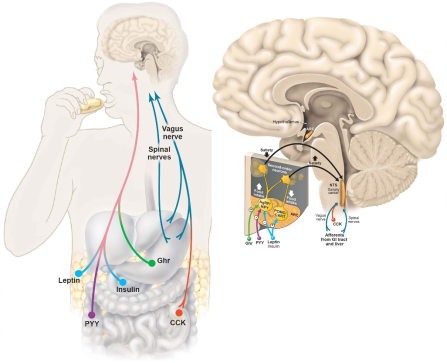
图2. 食欲调控激素以及中枢调控(Jean Marx., Science. 2003)
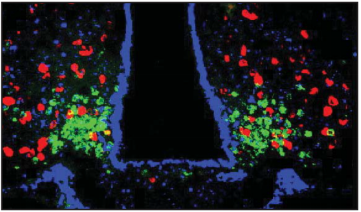
图3. ARC中AgRP神经元(绿色)和POMC神经元(红色)调控摄食行为(Jean Marx., Science. 2003)
饮食的神经环路调控机制
面对美味的食物,有时人们即使在饱食的情况下也会想要进食,这一点被认为是造成超重或者肥胖的重要原因。近几十年来,科学家们在寻找大脑中调控摄食的神经环路过程中做出不懈的努力,通过光遗传学、化学遗传学技术的结合,使科学研究能够在在体水平、自由活动动物上激活或抑制特定核团神经元亚群以及神经环路并观察是否影响摄食,进而判断参与调控摄食的中枢机制。研究发现,下丘脑的亚核团—外侧下丘脑(lateral hypothalamic, LH)是一个异质性的大脑区域,包含了多种表达不同基因的细胞亚群,如兴奋性神经元、抑制性神经元及其亚群等,它们可以精确地调控摄食行为 (Jennings et al., 2015)。在啮齿类动物上的研究结果显示,电刺激LH,即非特异性激活该核团多种细胞类型可显著增强进食行为;而毁损该核团则会导致摄食减少、体型消瘦 (Hoebel, 1965; Olds and Milner, 1954)。接下来的研究表明,选择性光遗传激活或抑制LH中抑制性神经元亚群可特异性地双向调控摄食行为 (Jennings et al., 2015)。除啮齿类动物外,在许多其它物种中(如爬行类、灵长类等)都发现了电刺激LH和特定的摄食活动相关的行为 (Molina-Borja and Gómez-Soutullo, 1989; Quaade et al., 1974)。这些研究表明了LH在调节生存本能行为(如摄食)中的重要性,同时跨物种研究高度一致的结果也提示了LH在调控摄食行为中高度保守的特性。
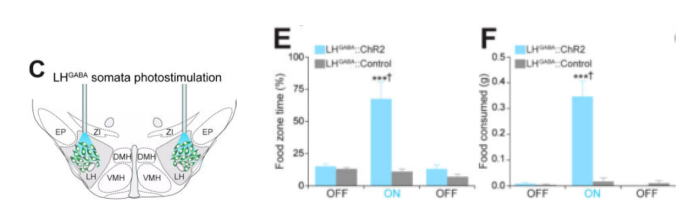
图4.光遗传调控外侧下丘脑抑制性神经元活性进而影响摄食行为(Jennings et al., Cell. 2015)
在神经环路层面,光遗传激活ARC中AgRP神经元至下丘脑室旁核(hypothalamic paraventricular nucleus,PVN)神经纤维末梢可以模拟光遗传激活ARC中AgRP神经元所引起的摄食行为,提示ARCAgRP至PVN的神经环路特异性调控摄食行为。此外,PVN投射至NTS的神经环路可释放催产素并影响后脑(脑桥和小脑)对肠源性饱腹信号CCK的响应,导致摄食减少 (Morton et al., 2014)(图5)。来自瑞士的Christian Lüscher课题组最新的研究综合利用光遗传学、病毒示踪、药理学、电生理等方法在神经环路以及分子水平揭示了摄食的调控机制,研究发现伏隔核D1神经元投射至LH的神经环路调控过度饮食行为 (Thoeni et al., 2020)。电生理记录显示在暴饮暴食的情况伏隔核D1至外侧下丘脑的信号传递被抑制,且这一抑制作用是由内源性大麻素一型受体(cannabinoid 1 receptor, CB1R)介导的。CB1R是内源性大麻素系统的重要组成成分,分布于伏隔核D1神经元投射至LH的突触末梢的CB1R激活被认为是诱导摄食的机制之一,CB1R被其激动剂药物(WIN55,212)激活后会促发随之而来的暴饮暴食(图6)。
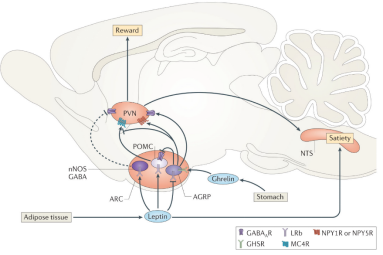
图5. 参与摄食调节的神经环路(Morton et al., Nat Rev Neurosci. 2014)
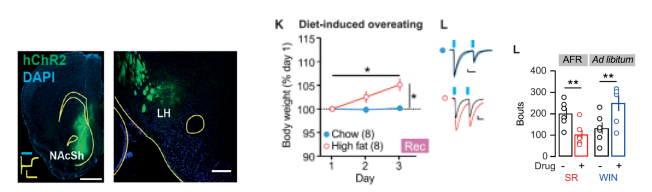
图6. 伏隔核D1神经元至LH神经环路上的CB1R信号通路调控暴饮暴食(Thoeni et al., Neuron. 2020)
结语
基于上述外周与中枢机制对摄食的动态调控作用,在正常个体中,体重和体脂含量通常是相当稳定的,这种调控被称为“能量内稳态”的生物过程。在现代社会,美味且高热量的食物唾手可得,过度饮食屡见不鲜,从而诱发代谢及相关疾病的发生。对摄食中枢调控机制的解析可以帮助我们寻找肥胖干预的有效手段及治疗肥胖相关疾病的新靶点,帮助我们更健康地生活。
参考文献
1. Marx J. Cellular warriors at the battle of the bulge. Science. 2003 Feb 7;299(5608):846-9. doi: 10.1126/science.299.5608.846. PMID: 12574615.
2. Jennings JH, Ung RL, Resendez SL, Stamatakis AM, Taylor JG, Huang J, Veleta K, Kantak PA, Aita M, Shilling-Scrivo K, Ramakrishnan C, Deisseroth K, Otte S, Stuber GD. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015 Jan 29;160(3):516-27. doi: 10.1016/j.cell.2014.12.026. PMID: 25635459; PMCID: PMC4312416.
Abstract
Optimally orchestrating complex behavioral states, such as the pursuit and consumption of food, is critical for an organism's survival. The lateral hypothalamus (LH) is a neuroanatomical region essential for appetitive and consummatory behaviors, but whether individual neurons within the LH differentially contribute to these interconnected processes is unknown. Here, we show that selective optogenetic stimulation of a molecularly defined subset of LH GABAergic (Vgat-expressing) neurons enhances both appetitive and consummatory behaviors, whereas genetic ablation of these neurons reduced these phenotypes. Furthermore, this targeted LH subpopulation is distinct from cells containing the feeding-related neuropeptides, melanin-concentrating hormone (MCH), and orexin (Orx). Employing in vivo calcium imaging in freely behaving mice to record activity dynamics from hundreds of cells, we identified individual LH GABAergic neurons that preferentially encode aspects of either appetitive or consummatory behaviors, but rarely both. These tightly regulated, yet highly intertwined, behavioral processes are thus dissociable at the cellular level.
3. Thoeni S, Loureiro M, O'Connor EC, Lüscher C. Depression of Accumbal to Lateral Hypothalamic Synapses Gates Overeating. Neuron. 2020 Jul 8;107(1):158-172.e4. doi: 10.1016/j.neuron.2020.03.029. Epub 2020 Apr 24. PMID: 32333845.
Abstract
Overeating typically follows periods of energy deficit, but it is also sustained by highly palatable foods, even without metabolic demand. Dopamine D1 receptor-expressing medium spiny neurons (D1-MSNs) of the nucleus accumbens shell (NAcSh) project to the lateral hypothalamus (LH) to authorize feeding when inhibited. Whether plasticity at these synapses can affect food intake is unknown. Here, ex vivo electrophysiology recordings reveal that D1-MSN-to-LH inhibitory transmission is depressed in circumstances in which overeating is promoted. Endocannabinoid signaling is identified as the induction mechanism, since inhibitory plasticity and concomitant overeating were blocked or induced by CB1R antagonism or agonism, respectively. D1-MSN-to-LH projectors were largely non-overlapping with D1-MSNs targeting ventral pallidum or ventral midbrain, providing an anatomical basis for distinct circuit plasticity mechanisms. Our study reveals a critical role for plasticity at D1-MSN-to-LH synapses in adaptive feeding control, which may underlie persistent overeating of unhealthy foods, a major risk factor for developing obesity.
4. Morton GJ, Meek TH, Schwartz MW. Neurobiology of food intake in health and disease. Nat Rev Neurosci. 2014 Jun;15(6):367-78. doi: 10.1038/nrn3745. PMID: 24840801; PMCID: PMC4076116.
Abstract
Under normal conditions, food intake and energy expenditure are balanced by a homeostatic system that maintains stability of body fat content over time. However, this homeostatic system can be overridden by the activation of 'emergency response circuits' that mediate feeding responses to emergent or stressful stimuli. Inhibition of these circuits is therefore permissive for normal energy homeostasis to occur, and their chronic activation can cause profound, even life-threatening, changes in body fat mass. This Review highlights how the interplay between homeostatic and emergency feeding circuits influences the biologically defended level of body weight under physiological and pathophysiological conditions.
—— 作者介绍 ——
 |
刘 雪 生物学专业,中国科学院大学2017级博士研究生 培养单位:中国科学院深圳先进技术研究院 导师:王立平 研究员、王枫 副研究员 研究方向:视觉本能恐惧的神经环路调控机制 |
